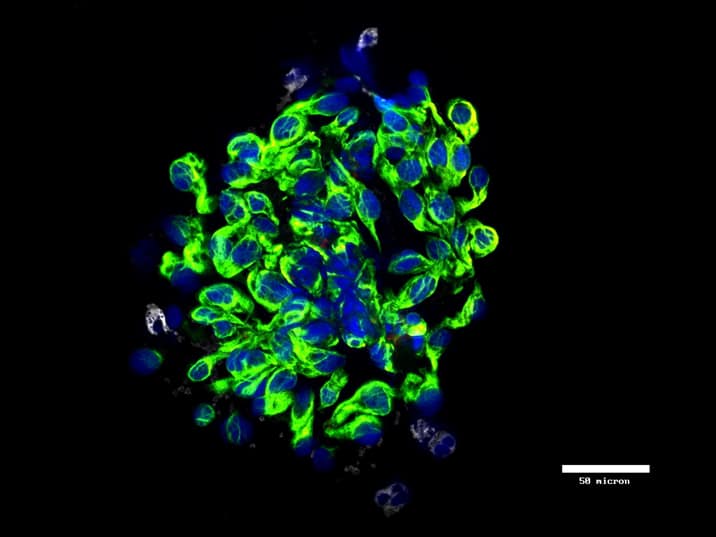
Patented microfluidic platform for intact CTC harvest
Capture and harvest circulating tumor cells (CTCs) from cancer patient blood for user-validated analysis.
Benefits of the Parsortix PC1 System
Provides access to biologically and clinically relevant, viable CTCs, including epithelial and mesenchymal phenotypes
Captures CTC and CTC clusters from whole blood, eliminating the need for sample pre-processing
Easy to operate system requiring minimal user intervention
Allows comprehensive analysis options, including cell morphology, DNA, RNA and protein analysis
Provides a CTC enriched sample compatible with single cell sorting for subsequent molecular analysis
Speak to us about how the Parsortix PC1 System could benefit your laboratory
Contact usLiquid biopsy is an emerging approach to cancer management that provides repeatable access to a tumour sample without the need for an invasive, and potentially dangerous solid tissue biopsy procedure.1
Download brochure Product intended useContact us to discuss how the Parsortix PC1 system can help with your laboratory’s liquid biopsy needs
Contact us
“As a breast cancer surgeon, I am very enthusiastic about the potential of liquid biopsy. Our pilot data shows that potentially the same information can be obtained from a simple blood test using the Parsortix® system as from an invasive tissue biopsy and indeed may be advantageous over invasive tissue biopsies in regard to the diverse sites of metastatic disease.”
Julie E. Lang
Chief of Breast Surgery, Cleveland Clinic. Formerly, Director, USC Breast Cancer Program, Associate Professor of Surgery, Norris Comprehensive Cancer Center, University of Southern California
References
- Cortés-Hernández LE, Eslami-S Z, Alix-Panabières C. Liquid Biopsy to Detect Circulating Tumor Cells: Is It Ready for a Value Proposition in Laboratory Medicine?. The Journal of Applied Laboratory Medicine. 2020 Sep;5(5):1027-37. NCCN Clincial Practice Guidelines in Oncology (NCCN Guidelines®), Breast Cancer. Version 2.2022, December 20, 2021.
- Carlson RW, et al, Metastatic breast cancer, version 1.2012: featured updates to the NCCN guidelines. Journal of the National Comprehensive Cancer Network. 2012 Jul 1;10(7):821-9
- Criscitiellio C, et al. Biopsy confirmation of metastatic sites in breast cancer patients: clinical impact and future perspectives. Breast Cancer Res 2014; 16(2):205-12
- Ring A, et al. Circulating Tumor Cell Transcriptomics as Biopsy Surrogates in Metastatic Breast Cancer. Annals of surgical oncology. 2022 May;29(5):2882-94.